Tatsuya Usui
| Position | Associate Professor |
|---|---|
| Affiliation | Institute of Agriculture |
| URL | https://kenkyu-web.tuat.ac.jp/profile/en.1b243833d32fbd159596fa7f10dd0003.html |
【LIFE SCIENCE】 Usui Team
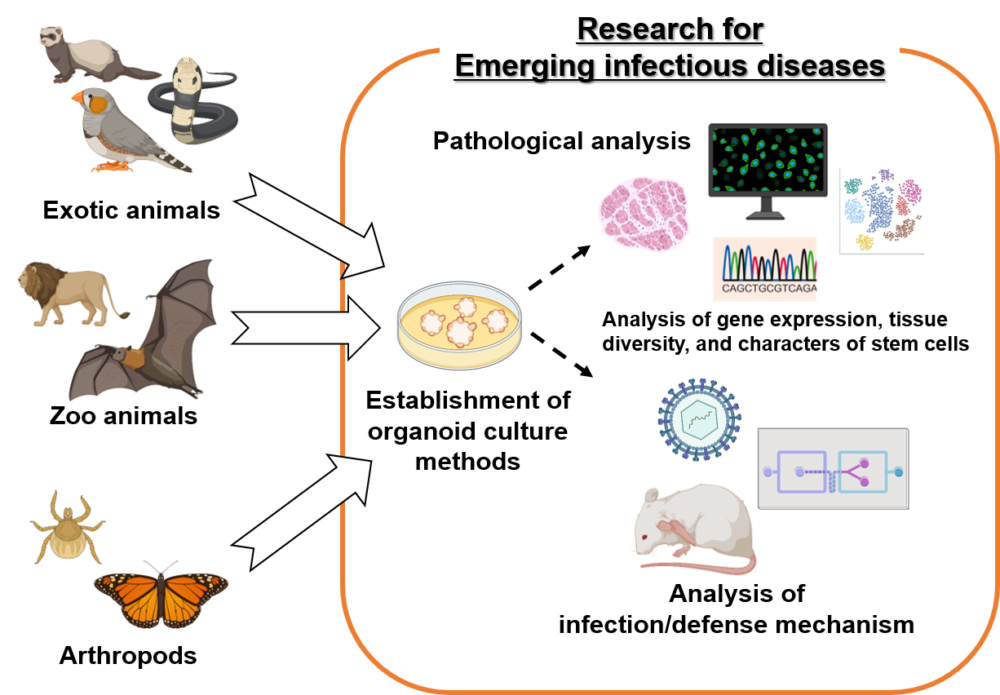
Recently, organoid culture has been developed as a method to culture biological tissues in three dimensions on a dish. The organoid culture method has been shown to be capable of reproducing the diversity and gene expression profiles of living tissues, including stem cells, and is being applied worldwide to various studies including cancer and infectious diseases. In this study, we have established an organoid culture method using companion animals such as ferrets, reptiles, and birds, zoo animals such as lions, and arthropod cells such as butterflies and ticks, by applying the technology we have cultivated through the production of 3D cultures derived from various animal cells. We will establish organoid culture methods using companion animals such as small birds, zoo animals such as lions, and arthropod cells such as butterflies and ticks, and clarify their usefulness as tools for pathological analysis and research on new infectious diseases transmitted by these animals and plants.

| Position | Associate Professor |
|---|---|
| Affiliation | Institute of Agriculture |
| URL | https://kenkyu-web.tuat.ac.jp/profile/en.1b243833d32fbd159596fa7f10dd0003.html |

| Affiliation | King Faisal University (Saudi Arabia) |
|---|---|
| Division / Department | College of Veterinary Medicine |
| Position | Professor |
| URL | https://scholar.google.com/citations?user=BOND2pcAAAAJ&hl=en |

| Affiliation | University of Birmingham (U.K.) |
|---|---|
| Division / Department | Division of Cardiovascular Sciences |
| Position | Bioinformatician |
| URL | https://scholar.google.com/citations?user=nIqPksEAAAAJ&hl=en |
Tsutomu Omatsu (Institute of Agriculture / Associate Professor)
Satoshi Koyama (Institute of Agriculture / Associate Professor)
このページの上部へ